
Sulfur oxides(SOx) - particularly sulfurdioxide, a chemical compound with the formula SO2. SO2 is produced by volcanoes and in various industrial processes. Coal and petroleum often contain sulfur compounds, and their combustion generates sulfur dioxide. Further oxidation of SO2, usually in the presence of a catalyst such as NO2, forms H2SO4, and thusacid rain.[2] This is one of the causes for concern over the environmental impact of the use of these fuels as power sources.


Nitrogen oxides(NOx) - Nitrogen oxides, particularly nitrogen dioxide, are expelled from high temperature combustion, and are also produced during thunderstorms by electric discharge. They can be seen as a brown haze dome above or a plume downwind of cities. Nitrogen dioxide is a chemical compound with the formula NO2. It is one of several nitrogen oxides. One of the most prominent air pollutants, this reddish - brown toxic gas has a characteristic sharp, biting odor .
Volatile organic compounds(VOC) - VOCs are a well - known outdoor air pollutant. They are categorized as either methane (CH4) or non - methane (NMVOCs). Methane is an extremely efficient greenhouse gas which contributes to enhanced global warming. Other hydrocarbon VOCs are also significant greenhouse gases because of their role in creating ozone and prolonging the life of methane in the atmosphere. This effect varies depending on local air quality. The aromatic NMVOCs benzene, toluene and xylene are suspected carcinogens and may lead to leukemia with prolonged exposure. 1,3 - butadiene is another dangerous compound often associated with industrial use.


Carbon monoxide (CO) - CO is a colorless, odorless, toxic yet non - irritating gas. It is a product by incomplete combustion of fuel such as natural gas, coal or wood. Vehicular exhaust is a major source of carbon monoxide.
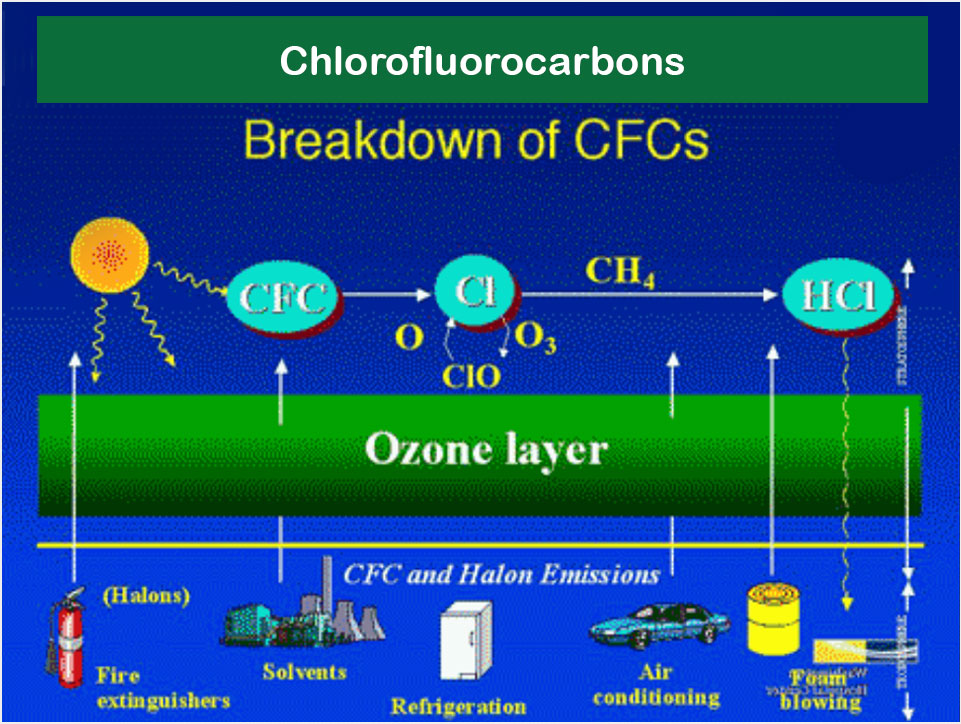
Chlorofluorocarbons (CFCs) harmful to the ozone layer; emitted from products are currently banned from use. These are gases which are released from air conditioners, refrigerators, aerosol sprays, etc. CFC's on being released into the air rises to stratosphere. Here they come in contact with other gases and damage the ozone layer. This allows harmful ultraviolet rays to reach the earth's surface. This can lead to skin cancer, disease to eye and can even cause damage to plants.

Ammonia (NH3) - emitted from agricultural processes. Ammonia is a compound with the formula NH3. It is normally encountered as a gas with a characteristic pungent odor. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to foodstuffs and fertilizers. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceuticals. Although in wide use, ammonia is both caustic and hazardous. In the atmosphere, ammonia reacts with oxides of nitrogen and sulfur to form secondary particles.
